Engineering resilience in the brain
Compared to the monumental machines of science, things like the International Space Station or the Large Hadron Collider, the human brain doesn't look like much. However, this three-pound amalgam of squishy cells is one of the most complicated and complex structures in the known universe.
With hundreds of billions of neurons, each with its own inner world of organelles and molecular components, understanding the fundamental wiring of the brain is a major undertaking, one that has received a commitment of at least $100 million worth of federal funding from the National Science Foundation (NSF), the National Institutes of Health and the Defense Advanced Research Projects Agency.
And with all of the brain's interconnected structures, protecting or repairing this complicated machine means thinking like an engineer.
"The idea is really quite simple," says Vivek Shenoy, an NSF-supported professor of materials science and engineering at the University of Pennsylvania's School of Engineering and Applied Science. "All of the mechanical properties of cells come from their cytoskeleton and the molecules within it. They're all reinforcing frames, like the frame in a building. Engineers design buildings and other structural objects to make sure they don't fail, so it's the same principle: structural engineering on a very, very small level."
Shenoy applies this approach to a problem very much in the public eye--traumatic brain injury. Even the mildest forms of TBI, better known as concussions, can do irreversible damage to the brain. More serious forms can be fatal.
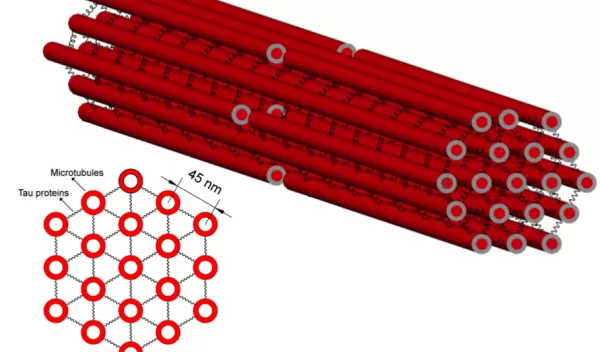
With a background in mechanical engineering and materials science, one might think that Shenoy's contribution to this problem involves designing new helmets or other safety devices. Instead, he and his colleagues are uncovering the fundamental math and physics behind one of the core mechanisms of the injury: swelling in axons caused by damage to internal structures known as microtubules. These neural "train tracks" transport molecular cargo from one end of a neuron to another; when the tracks break, the cargo piles up and produces bulges in the axons that are the hallmark of fatal TBIs.
Armed with a better understanding of the mechanical properties of these critical structures, Shenoy and his colleagues are laying the foundations for drugs that could one day bolster neurons' reinforcing frames, making them more resilient when faced with a TBI-inducing impact.
Train tracks and crossties
The first step toward this understanding was resolving a paradox: Why were the microtubules, the stiffest elements of the axons, the parts that were breaking when loaded with the stress of a blow to the head?
A recent finding from Shenoy's team shows that the answer rests with a critical brain protein known as tau, which is implicated in several neurodegenerative diseases, including Alzheimer's. If microtubules are like train tracks, tau proteins are the crossties that hold them together. The protein's elastic properties help explain why rapid movement of the brain, whether on a football field or a car crash, leads to TBI.
Shenoy's colleague Douglas Smith, professor of neurosurgery in Penn's Perelman School of Medicine and director of the Penn Center for Brain Injury and Repair, had previously studied the mechanical properties of axons, subjecting them to strains of different forces and speeds.
"What we saw is that with slow loading rates, axons can stretch up to at least 100 percent with no signs of damage," Smith said. "But at faster rates, axons start displaying the same swellings you see in the TBI patients. This process occurs even with relatively short stretches at fast rates."
To explain this rate-dependent response, Shenoy and Smith had to delve deeper inside the structure of microtubules. Based on Smith's work, other biophysical modelers had previously accounted for the geometry and elastic properties of the axon during a stretching injury, but they did not have good data for representing tau's role.
"You need to know the elastic properties of tau," Shenoy said, "because when you load the microtubules with stress, you load the tau as well. How these two parts distribute the stress between them is going to have major impact on the system as a whole."
Elastic properties
Shenoy and his colleagues had a sense of tau's elastic properties but did not have hard numbers until a 2011 experiment from a Swiss and German research team physically stretched out lengths of tau by plucking it with the tip of an atomic force microscope.
"This experiment demonstrated that tau is viscoelastic," Shenoy said. "Like Silly Putty, when you add stress to it slowly, it stretches a lot. But if you add stress to it rapidly, like in an impact, it breaks."
This behavior is because the strands of tau protein are coiled up and bonded to themselves in different places. Pulled slowly, those bonds can come undone, lengthening the strand without breaking it.
"The damage in traumatic brain injury occurs when the microtubules stretch but the tau doesn't, as they can't stretch as far," Shenoy said. "If you're in a situation where the tau doesn't stretch, such as what happens in fast strain rates, then all the strain will transfer to the microtubules and cause them to break."
With a comprehensive model of the tau-microtubule system, the researchers were able to boil down the outcome of rapid stress loading to equations with only a handful of variables. This mathematical understanding allowed the researchers to produce a phase diagram that shows the dividing line between strain rates that leave permanent damage versus ones that are safe and reversible.
Next steps
Having this mathematical understanding of the interplay between tau and microtubules is only the beginning.
"Predicting what kind of impacts will cause these strain rates is still a complicated problem," Shenoy said. "I might be able to measure the force of the impact when it hits someone's head, but that force then has to make its way down to the axons, which depends on a lot of different things.
"You need a multiscale model, and our work will be an input to those models on the smallest scale."
In the longer term, however, knowing the parameters that lead to irreversible damage could lead to better understanding of brain injuries and diseases and to new preventive measures. It may even be possible to design drugs that alter microtubule stability and elasticity of axons in traumatic brain injury; Smith's group has demonstrated that treatment with the microtubule-stabilizing drug taxol reduced the extent of axon swellings and degeneration after injuries in which they are stretched.
Ultimately, insights on the molecular level will be inputs to a more comprehensive view of the brain and its many hierarchies of organizations.
"When you're talking about something's mechanical properties, stiffness is what comes to mind," Shenoy said. "Biochemistry is what determines that stiffness in the brain's structures, but that's only at the molecular level. Once you build it up and formulate things at the appropriate scale, protecting the brain becomes more of a structural engineering problem."
Editor's Note: This Behind the Scenes article was first provided to LiveScience in partnership with the National Science Foundation.
