New technology could make biopsies a thing of the past
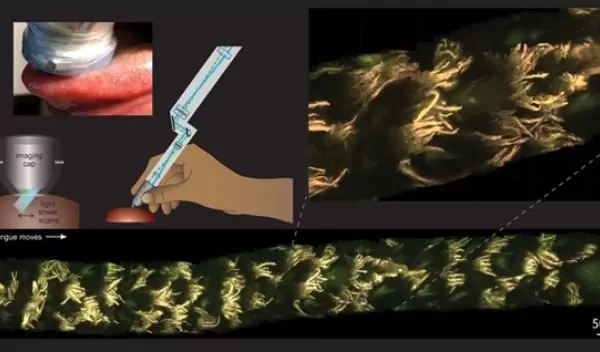
U.S. National Science Foundation grantee engineers at Columbia University have created MediSCAPE, a technology with possible surgical, diagnostic and therapeutic applications. MediSCAPE is a high-speed 3D microscope that provides real-time detailed images of live tissue cells. Instead of excising tissue for a biopsy, doctors could examine living tissue through this technology.
"The way biopsy samples are processed hasn't changed in 100 years," said Elizabeth Hillman, senior author of the study. "They are cut out, fixed, embedded, sliced, stained with dyes, positioned on a glass slide, and viewed by a pathologist using a simple microscope. That's why it can take days to hear news back about your diagnosis after a biopsy."
So the team developed an alternative to biopsies that captures real-time images. "The technology could give doctors real-time feedback about what type of tissue they are looking at without the long wait," Hillman explained. "This instant answer would let them make informed decisions about how best to cut out a tumor and ensure there is none left behind."
One major benefit of the approach is risk reduction, especially in delicate and difficult to biopsy areas like the brain, spinal cord, nerves and eyes. MediSCAPE also doesn't require the use of fluorescent dye, removing that limitation for some patients.
"Understanding whether tissues are staying healthy and getting good blood supply during surgical procedures is really important," said Hillman. "If we don't have to remove tissues to look at them, we can find many more uses for MediSCAPE, even to answer simple questions such as, 'What tissue is this?' or to navigate around nerves. These applications are important for robotic and laparoscopic surgeries where surgeons are more limited in their ability to identify and interact with tissues directly."
The team created a smaller format version of MediSCAPE that could be used by a surgeon in an operating room, and plans to conduct a larger clinical trial as well as to obtain FDA approval.
