Research shows how tissue's microscopic geometry affects spread of cancer
Oregon State University research has revealed a crucial mechanism behind one of humankind's most deadly physiological processes: the movement of malignant cells from one part of the body to another.
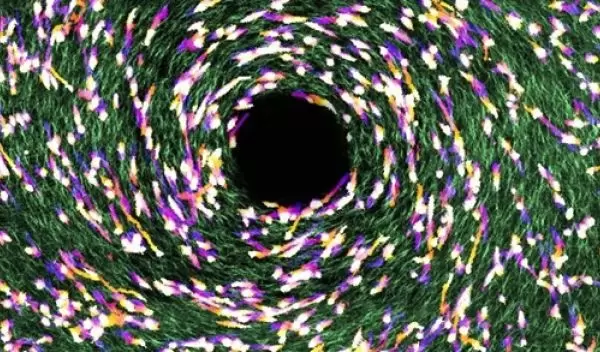
Published in Proceedings of the National Academy of Sciences, the U.S. National Science Foundation-funded study led by OSU biophysicist Bo Sun shows the role that tissues' microscopic geometry plays in cancer metastasis, the internal spreading of the disease that's responsible for 95% of all cancer deaths.
To develop drugs that effectively combat metastasis, it's important to understand what directs the process, Sun said. "Our results show that the level of tissue fiber alignment, particularly collagen fiber alignment, is a crucial part of what's happening."
Collagen is a protein that serves as the primary component of human connective tissue, which supports, protects and provides structure for other tissues and organs in the body. Collagen is also a key part of the extracellular matrix, the non-cellular part of tissues and organs that acts as a scaffold and performs important biochemical and biomechanical functions.
"Clinical studies have shown that the microscopic geometry of tissue is significantly correlated with the progression of breast cancers," Sun said. "Our study reveals the underlying biophysical mechanism. In the era of precision medicine, we think taking into account the physical properties of a patient's tissue can be critical for the prediction and treatment of metastatic disease."
The correlation is due to a cellular phenomenon known as "contact guidance," which is analogous to a back-country hiker trying to pick a route based on the contours of the terrain and the network of downed trees on the ground.
"In navigating the three-dimensional extracellular matrix, where the fibers aren't necessarily parallel, cells have to integrate multiple guidance cues that aren't always clear and sometimes are conflicting," Sun said. "Understanding the mechanisms and limitations of cell responses to imperfect guidance signals is pivotal for predicting and engineering cell behaviors and providing a patient with a precise diagnosis, prognosis and treatment."


