Research shows why cancer stops responding to kinase-blocking drugs and comes back stronger
More than 70 FDA-approved cancer drugs are kinase inhibitors, which work by blocking kinases — enzymes that add phosphate groups to molecules in the cell — and preventing the chemical activity necessary for signaling and growth in cancer cells. Kinase inhibitors can be very effective, but long-term, some patients experience aggressive recurrences that are more difficult to treat and resistant to the original drug.
A U.S. National Science Foundation-supported study by New York University researchers, published in Proceedings of the National Academy of Sciences, explains why cancers not only stop responding to kinase inhibitors but come back stronger, a finding that could inform which drugs oncologists use as a first-line treatment.
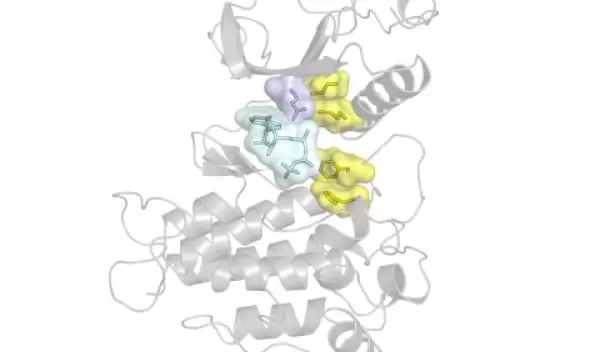
A leading cause of drug resistance — when a cancer no longer responds to a kinase inhibitor — is the emergence of genetic mutations, particularly those in a region in the kinase called the "gatekeeper" residue. The gatekeeper is deeply embedded in the kinase and allows or prevents access to an even-deeper hydrophobic (or water-repelling) pocket.
Proteins are made up of building blocks called amino acids. "Mutation of gatekeeper amino acid to a larger one blocks or partially hinders drugs' access to their target area, causing reduced effectiveness," says Engin Serpersu, a program director in NSF's Division of Molecular and Cellular Biosciences.
Because kinase inhibitors work by binding to this hydrophobic pocket, mutations to the gatekeeper residue block a drug's access, reducing its efficacy. But gatekeeper mutations also do something else that, according to the NYU researchers, may be even more important: they make kinases more active.
The findings could inform how clinicians choose which first-line cancer treatment to use — and whether a cocktail of drugs instead may be more effective at preventing recurrence.
The researchers are also considering how these findings could be used in the development of novel cancer therapeutics. One avenue they're exploring is finding locations in the kinase other than the hydrophobic pocket for drugs to bind to, given not only the prospect of gatekeeper mutations but also that these pockets look so similar in the roughly 500 different types of human kinases, which limits the chances that a drug can precisely target certain kinases.
