The stars within us
Humans have always looked to the stars and studied them. Over the past century, science has revealed the fundamental role stars play for nearly everything in existence, including the elements on the Periodic Table.
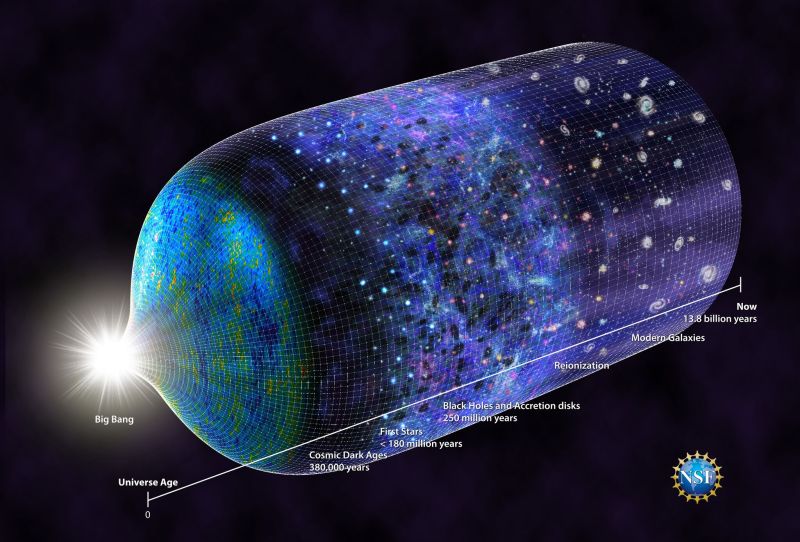
The birth, life and death of every star creates and disseminates the elements of the Periodic Table throughout the universe, a cycle that began nearly 14 billion years ago and repeats continuously today.
Without it, the Earth and everything on it – air, water, soil, plants, wildlife, and human life – would not exist.
Birth of stars and the first elements
Within the first three minutes following the Big Bang, the fundamental building blocks of matter formed and merged into the first element–hydrogen. Within a few hundred million years after the Big Bang, clouds of hydrogen gas condensed into the first stars. In the cores of those stars, intense heat and pressure fused hydrogen atoms to form helium and lithium.
Recently, astronomers from several U.S.-based universities detected a signal from the birth of those early stars. Since the stars are too distant to be seen with telescopes, the astronomers searched for indirect evidence, such as a tell-tale change in the background electromagnetic radiation that permeates the universe, called the cosmic microwave background.
Supported for more than a decade by the U.S. National Science Foundation, researchers placed a radio antenna not much larger than a refrigerator in the Australian desert and found clear evidence of these massive blue stars.
More chaos, more elements
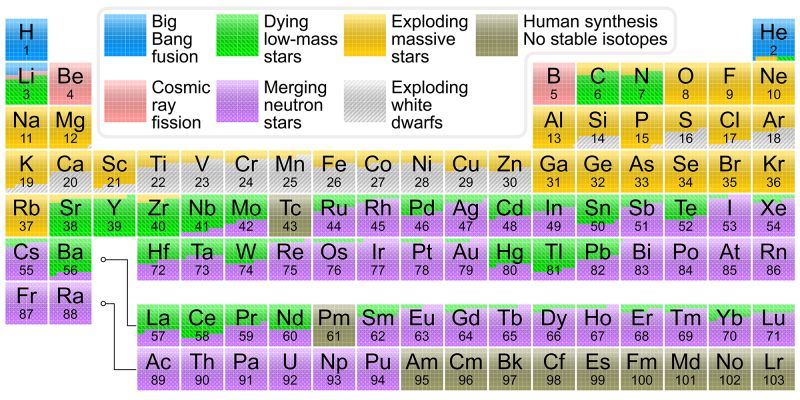
The normal functions of a star—those that make it shine brightly and burn at temperatures of thousands of degrees—create the simplest and lightest elements. Creation of heavier elements requires more extreme environments, usually triggered by the end of a star’s life.
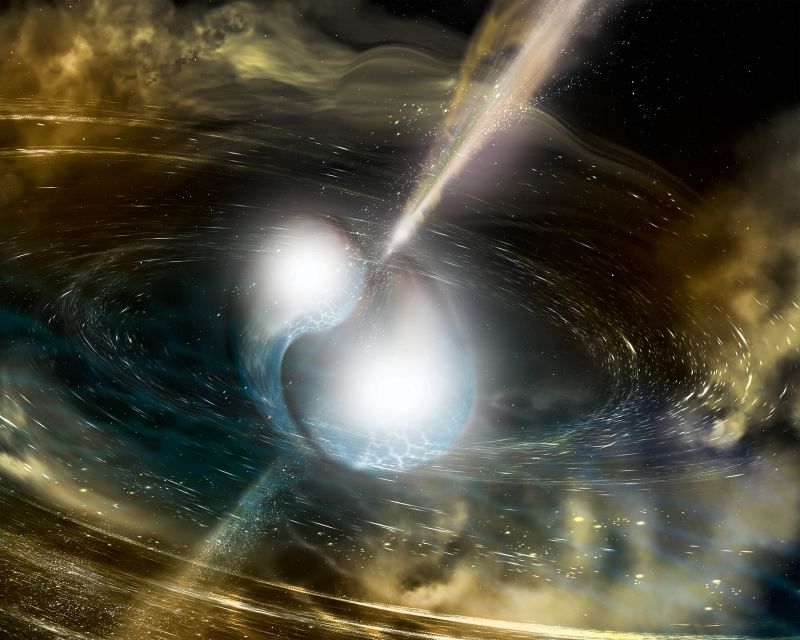
After the hydrogen in a star's core is exhausted, the star fuses helium to form progressively heavier elements, such as carbon and iron. As this fuel runs out, the star either explodes into a supernova, seeding the universe with those elements, or violently collapses, creating neutron stars and black holes. In such violent implosions, star collisions, and the extreme environments around black holes, the heavier elements are forged and then spread far across interstellar space.
In 2017, for the first time in history, researchers using the twin detectors of NSF’s Laser Interferometer Gravitational-Wave Observatory detected gravitational waves created by the collision of two neutron stars. The researchers worked with the Europe-based Virgo gravitational wave detector and some 70 ground- and space-based telescopes across the globe to track and record the gamma radiation, X-rays, light, and radio waves that cascaded from the explosion.
The observations revealed signatures of recently synthesized elements, including gold and platinum, solving a decades-long mystery of how nearly half of all elements heavier than iron are produced.
Some of the heaviest elements, such as uranium, are forged near black holes and in the powerful jets that can emanate from them, such as those that surge away from “feeding” black holes, like blazars, an active galactic nucleus with a relativistic jet composed of ionized matter.
The NSF-supported Event Horizon Telescope presented the first direct visual evidence of a supermassive black hole in 2019, and NSF’s Ice Cube detector has worked with collaborating observatories to trace a cosmic neutrino to its blazar source.
These extreme environments in space are where the heaviest elements are formed, but because they have such short half-lives, scientists have yet to directly witness their formation, and they have not survived to be found on Earth today.
This is where researchers in the laboratory have built upon what we have learned from studying the cosmos.
Filling the Periodic Table
On Earth, ancient cultures were first to isolate a handful of elements, such as copper and mercury, though in recent centuries, scientists have identified and isolated more than 100 more. They are categorized using the Periodic Table—first published in 1869 by Russian chemist Dmitri Mendeleev. The initial Periodic Table contained 28 elements, and Mendeleev predicted the existence of unidentified elements, leaving gaps for future scientists to fill.
Laboratory experiments have expanded the Periodic Table to include 118 known elements. For some, particularly the heaviest, they were only discovered when physicists crafted them from the fusion of lighter elements. The heaviest known element is oganesson, which holds 118 protons in its nucleus, although only for fractions of a millisecond.
Like the stars that constantly recycle and distribute elements throughout space, researchers in all disciplines continue their efforts to expand the Periodic Table and deepen the understanding of the atoms from which we are constructed. This is an ongoing process, and future generations of scientists are just now making their initial observations or conducting their first experiments that will expand the knowledge about the universe and ourselves.